一期臨床試驗聯合科學委員會 (Joint Scientific Committee for Phase 1 Clinical Trials, JSC) 由香港大學 (港大)、香港中文大學(中大)及醫院管理局(醫管局)共同成立,以支援其研究倫理委員會進行創新試驗用藥物的一期臨床試驗科學評估。JSC由以上三個機構透過稱為臨床研究機構要求協調聯盟(Consortium on Harmonization of Institutional Requirements for Clinical Research, CHAIR) 的三方聯合組織管理,並根據JSC的SOP運作。JSC旨在透過對創新試驗用藥物的特性、試驗設計、臨床前數據及初步人體數據進行精確的科學評估,保障研究參加者的安全,以及確保一期臨床試驗的科學有效性。
一期临床试验联合科学委员会 (Joint Scientific Committee for Phase 1 Clinical Trials, JSC) 由香港大学 (港大)、香港中文大学(中大)及医院管理局(医管局)共同成立,以支援其研究伦理委员会进行创新试验用药物的一期临床试验科学评估。JSC由以上三个机构透过称为临床研究机构要求协调联盟(Consortium on Harmonization of Institutional Requirements for Clinical Research, CHAIR) 的三方联合组织管理,并根据JSC的SOP运作。JSC旨在透过对创新试验用药物的特性、试验设计、临床前数据及初步人体数据进行精确的科学评估,保障研究参加者的安全,以及确保一期临床试验的科学有效性。
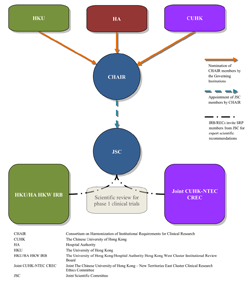
The Joint Scientific Committee for Phase 1 Clinical Trials (JSC) was established jointly by HKU, The Chinese University of Hong Kong (CUHK) and the Hospital Authority (HA) for supporting their institutional review board/research ethics committee in performing scientific evaluation of phase 1 clinical trials on new investigational drugs. It is governed jointly by the three organizations through a tripartite consortium namely the Consortium on Harmonization of Institutional Requirements for Clinical Research (CHAIR), and operates in accordance with the JSC’s SOP. Its mission is protecting the safety of study participants and ensuring the scientific validity of phase 1 clinical trials through rigorous scientific evaluation of the properties of new investigational drugs, trial designs, pre-clinical data and initial human data.
就向HKU/HA HKW IRB提交以供審閱的一期試驗申請而言,其一期專責小組將邀請三名JSC成員出任科學審查員,根據SOP of the JSC及一期臨床試驗倫理監督及科學評估指引 (Guideline on Ethics Oversight and Scientific Evaluation of Phase 1 Clinical Trials)對試驗進行科學評估。一期專責小組將屆時考慮科學審查員的科學意見,並就申請作出決定或提供意見。
就向HKU/HA HKW IRB提交以供审阅的一期试验申请而言,其一期专责小组将邀请三名JSC成员出任科学审查员,根据SOP of the JSC及一期临床试验伦理监督及科学评估指引 (Guideline on Ethics Oversight and Scientific Evaluation of Phase 1 Clinical Trials)对试验进行科学评估。一期专责小组将届时考虑科学审查员的科学意见,并就申请作出决定或提供意见。
For instance, for a phase 1 trial application submitted to HKU/HA HKW IRB for review, its Phase 1 Panel will invite three members of the JSC as scientific reviewers to perform scientific evaluation of the trial based on the SOP of the JSC and Guideline on Ethics Oversight and Scientific Evaluation of Phase 1 Clinical Trials. The Phase 1 Panel will then consider the scientific opinions from the scientific reviewers and make a decision or give opinions on the application.
